Cement as sprayed concrete constituent material in Mining Industry
Cements shall fulfill the requirements of EN197 or alternatively comply with the national standards or regulations valid in the place of use of the sprayed concrete. Only cement with recognized suitability for sprayed concrete applications shall be used.
Cement Production
In the following text, the cement production process is summarized as described in [24].
“Cement is typically made from limestone and clay or shale. These raw materials are extracted from the quarry, crushed to a very fine powder and then blended in the correct proportions.
This blended raw material is called the ‘raw feed’ or ‘kiln feed’ and is heated in a rotary kiln where it reaches a temperature of about 1400 °C to 1500 °C. In its simplest form, the rotary kiln is a tube up to 200 meters long and perhaps 6 meters in diameter, with a long flame at one end. The raw feed enters the kiln at the cool end and gradually passes down to the hot end, then falls out of the kiln and cools down.
The material formed in the kiln is described as ‘clinker’ and is typically composed of rounded nodules between 1mm and 25mm across.
After cooling, the clinker may be stored temporarily in a clinker store, or it may pass directly to the cement mill.
The cement mill grinds the clinker to a fine powder. A small amount of gypsum – a form of calcium sulfate – is normally ground up with the clinker. The gypsum controls the setting properties of the cement when water is added.”
The chemical composition of gypsum however is dependent on the temperature. Above 170 °C it is calcium sulfate (CaSO4) called anhydrate, above 105 °C, it is described as CaSO4 ∙ ½H2O, whereas normal gypsum contains more crystal water with a chemical formula of CaSO4 ∙ 2 H2O.
![Cement production process according to [25]](https://assets.construction-chemicals.mbcc-group.com/en-za/cement%20production%20process%20according%20to%2025.jpg)
Cement Types and Constituents
The 27 types in the list of common cements and their notation are provided by EN-197-1, see [1]. They are grouped into five main cement types as displayed in the following table. The detailed composition of each of the 27 products in the family of common cements shall be in accordance with EN 197-1.
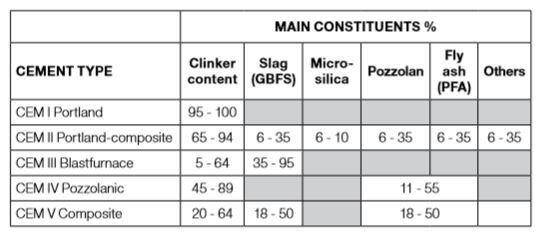
Table 3-1: Cement types and their main constituents
As described in EN 14487-1 [32]: “The type of cement shall be specified, taking into account the influence of current temperature and heat evaluation on required workability time, the requirement on strength development and final strength as well as the current curing conditions. If required, it shall be checked by means of an appropriate method.
For permanent structures, the environmental conditions to which the sprayed concrete is exposed shall be in accordance with EN 206-1, as well as precautions regarding resistance to alkali-silica reactions according to EN 206-1.”
Since sprayed concrete is intended to develop fast early-strength performance, a high clinker content is inevitable to achieve an immediate reaction with set accelerators. Depending on the cement types selected on site, it is necessary to choose the best performing accelerator. The admixture industry offers a full range of specialty products.
Cement Phases
Portland cement is made by heating a mixture of limestone and clay, or other materials of similar bulk composition and sufficient reactivity, ultimately to a temperature of about 1450 °C. The clinker is then mixed with calcium sulfate and finely ground to make the cement (see [2] to [5]). The clinker normally contains four major mineral phases, shown in Figure 3-2, see [6].
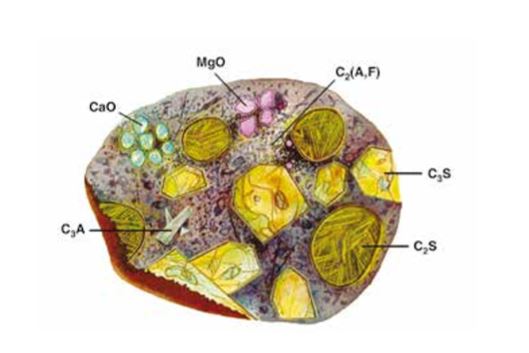
Figure 3-2: Representation of a cross-section of a cement grain [5] (C3S-Ca3SiO5 , C2S-Ca2SiO4 , C3A-Ca3 Al2O6 , C2(A,F)- Ca2 AlFeO5 )
Alite
Alite is the most important mineral phase of all normal Portland cement clinkers, of which it constitutes 50–70 %. It is tri-calcium silicate (Ca3SiO5 or C3S) modified in composition and crystal structure by ionic substitutions. It reacts relatively quickly with water, and in normal Portland cement is the most important of the constituent phases for strength development; at ages up to 28 days, it is by far the most important.
Belite
Belite constitutes 15–30 % of normal Portland cement clinkers. It is dicalcium silicate (Ca2SiO4 or C2S) modified by ionic substitutions and normally present wholly or largely as the ß- polymorph. It reacts slowly with water, thus contributing little to the strength during the first 28 days, but substantially to the further increase in strength that occurs at later ages.
Aluminate
Aluminate constitutes 5–10 % of most normal Portland cement clinkers. It is tricalcium aluminate (Ca3Al2O6 or C3A), substantially modified in composition and sometimes also in structure by ionic substitutions. It reacts rapidly with water and can cause undesirably rapid setting unless a set-controlling agent, usually gypsum, is added.
Ferrite
Ferrite makes up 5–15 % of normal Portland cement clinkers. It is tetracalcium aluminoferrite (Ca2AlFeO5 or C2AF), substantially modified in composition by variation in Al/Fe ratio and ionic substitutions. The rate at which it reacts with water appears to be somewhat variable, perhaps due to differences in composition or other characteristics, but in general is high initially and low or very low at later ages.
Cement Hydration
Chemical reactions of cement hydration
In cement chemistry, the term “hydration” denotes the totality of the changes that occurs when anhydrous cement, or one of its constituent phases, is mixed with water. The main chemical reactions of cement clinker with water are described in the following based on [2]. It should be considered that the individual steps of cement hydration generate specific amounts of heat, which contribute to setting and hardening.
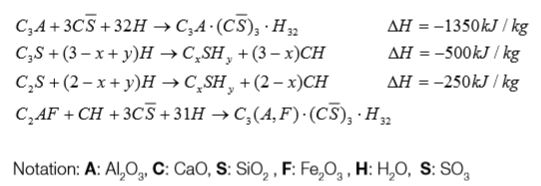
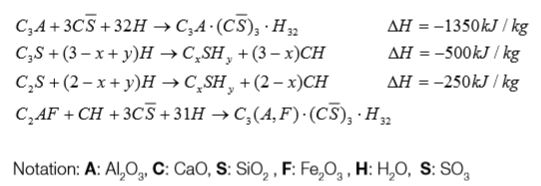
Gypsum is added to clinker to avoid uncontrolled setting, the so-called false or flash set.
Microstructure development: crystal growth
The main features of the development of microstructures in cement paste are by Scrivener (see [4] and [7]). There are plenty of research findings on the kinetics and modeling of cement hydration (see [2] to [12]).
Ettringite (see Figure 3-3), occurs as hexagonal prismatic crystalline; it is usually the first hydration product to crystallize because of the high sulfate/aluminate ratio in the solution phase during the first hour of hydration. In normal Portland cements which contain 5 to 6 % gypsum, the precipitation of ettringite contributes to stiffening (loss of consistency), setting (solidification of the paste) and early strength development.

Figure 3-3: Hexagonal prismatic ettringite crystals formed in cement paste, 10 min hydration
The calcium silicate hydrate phase, abbreviated as C-S-H, makes up 50 to 60 % of the volume of solids in a completely hydrated Portland cement paste and is, therefore, the most important phase determining the properties of the paste. The acceleratory period of hydration is characterized by rapid formation/crystallization of C-S-H and calcium hydroxide. The Ca/Si ratio of C-S-H varies between 1.5 and 2.0 and the structural water content varies even more. The morphology of C-S-H can be in the form of sheets (C-S-H(I)) and fibers (C-S-H(II)). Both of these are virtually amorphous to X-rays.
Portlandite, calcium hydroxide crystals, constitutes 20 to 25 % of the volume of solids in the hydrated paste. It tends to form large crystals with distinctive hexagonal-prism morphology. Compared to C-S-H, the strength contributing potential of calcium hydroxide is limited as a result of a considerably lower surface area.
Contributions of hydration products to strength development
Figure 3-4 shows the contributions of hydration products to strength development in pure cement compound cement pastes by time. The hydration process is a complex system consisting of many parallel or sequential chemical reactions. Intermediates and products are difficult to describe in chemical terms. During the last few decades, science and industry have undertaken successful efforts to understand the hydration processes and its consequences for concrete technology.
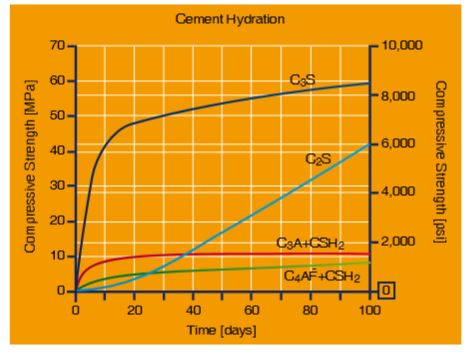
Figure 3-4: Development of compressive strength in pure cement compound pastes (source: Mindess et al, 2003)
Better understanding of the contributions shown in Figure 3-4, has assisted the admixture industry in the development of more effective and specific admixtures, e.g. for faster strength development.
Typical characteristics of cement related to sprayed concrete
In order to obtain satisfactory results for sprayed concrete, not only the cement type has to be considered. In addition, there are a number of other key factors such as clinker content, fineness (Blaine value), C3A and gypsum contents. These factors are responsible for rapid setting and early strength development.
In the following table examples of typical parameters required for sprayed concrete are shown. Lower C3A and gypsum contents than the values shown on Table 3-2 negatively influence setting and strength development performances.
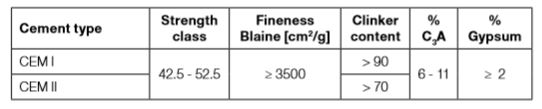
Table 3-2: Some key parameters of most commonly used cements for sprayed concrete
The minimum cement content for a particular environmental condition (exposure class) shall be 300 kg/m3 and as required by EN 206 or National Standards and regulations valid in the place of use.
It is important to understand that fineness and strength class of the selected cement play an important role for the setting and strength development of sprayed concrete. A finer cement of strength class 52.5 is more reactive than a coarser cement of strength class 32.5. The following table summarizes the specifications and typical setting times of different cement types.
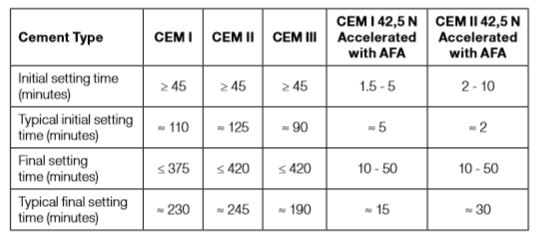
Table 3-3: Setting times (with and without set accelerators) of cements based on strength classes
Economical and environmental megatrend
As reported by the Carnegie Mellon University, Center for Building Performance and Diagnostics (CBPD) [40]:
“Understanding and observing global megatrends provides a concrete first hint about where to focus in the future of construction materials. The key trends, relevant for the construction industry are ongoing urbanization, growth and aging of the world population, global water crisis, concentration of wealth and poverty and growing use and importance of alternative energy sources.”
Sustainability in construction or development that meets the needs of the present generation without compromising the ability of future generations to meet their own needs will disrupt the industry like no other development has ever done before. Considering these factors, important criteria for the future assessment of sprayed concrete usage are summarized in Table 3-4.

Table 3-4: Important criteria for future assessment of sprayed concrete usage and related aspects/requirements
As reported by the Carnegie Mellon University, Center for Building Performance and Diagnostics (CBPD):
“Concrete will remain the most important construction material. Compared to plastic and steel, concrete is already a rather ‘green’ construction material, due to its comparably low carbon footprint [concrete: ~ 120 kg CO2 / ton vs. plastic: 798 kg CO2 / ton]. The major trends are the drastic decrease in clinker content, the gradual shift into higher strength classes and a stronger promotion of thermal mass of concrete to fight lightweight construction.”
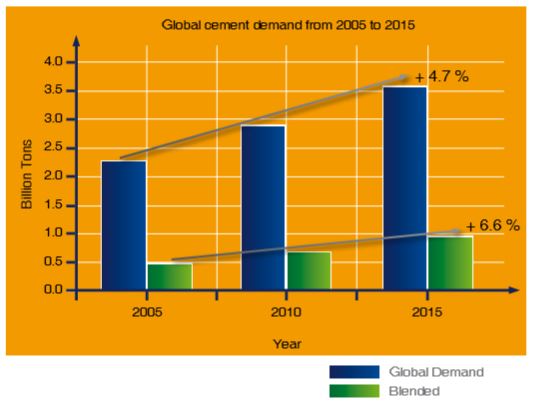
Regarding clinker and CO2 reduction, a trend has been observed towards blended cements. As shown in Figure 3-5, blended ratio increases more greatly than the overall total global demand for cement. This trend is expected to continue in coming years.

Figure 3-5: Global cement demand (billion tons) from 2005 and expectation towards 2015 (source: Fredonia)
The use of blended cements in sprayed concrete continues to increase in line with their overall use for construction. Using blended cements can result in reduced setting and early strength development in the sprayed concrete compared with mixes consisting of “pure” non-blended cements.

